Gas Chromatography–Mass Spectrometry (GC-MS): Principles, Technical Insights, and Applications
GC-MS, short for Gas Chromatography–Mass Spectrometry, is an analytical technique that combines the physical separation capabilities of gas chromatography (GC) with the molecular identification power of mass spectrometry (MS). It enables the separation, detection, and structural elucidation of volatile and semi-volatile organic compounds with exceptional sensitivity and specificity.Table of Contents
What Does GC-MS Stand For?
The acronymGC-MS stands for Gas Chromatography–Mass Spectrometry. It refers to the integration of two powerful analytical instruments: gas chromatography, which separates mixtures into individual compounds based on their volatility and interaction with the chromatographic column, and mass spectrometry, which detects and identifies molecules based on their mass-to-charge ratios (m/z).
This combined technique is commonly referred to in scientific literature and industry notes as GCMS. It is extensively used in analytical laboratories for qualitative and quantitative chemical analysis.
Fundamental Principles of GC-MS
GC-MS capitalizes on the complementarity of two distinct yet synergistic principles:- Gas Chromatography (GC): This technique separates components of a mixture by passing a vaporized sample through a capillary column coated with a stationary phase. Components partition between the mobile gas phase and stationary phase, leading to differential retention times.
- Mass Spectrometry (MS): This technique ionizes chemical species, generating charged molecular fragments. The ions are then sorted and detected according to their mass-to-charge ratios (
m/z), producing a mass spectrum which serves as a molecular fingerprint.
Detailed Workflow of GC-MS Analysis
- Sample Injection and Vaporization: The analyte is introduced via a heated injection port where it rapidly vaporizes without decomposition.
- Chromatographic Separation: The vaporized sample is carried by an inert gas (e.g., helium, nitrogen) through a capillary column housed in a temperature-controlled oven. The separation is governed by the compounds’ volatility and affinity for the stationary phase coating the column walls.
- Transfer Interface: The column effluent enters the mass spectrometer through a heated transfer line, preventing condensation and maintaining sample integrity.
- Ionization: Typically, electron ionization (EI) is used, bombarding molecules with electrons (~70 eV), inducing fragmentation into reproducible charged species.
- Mass Analysis: The ionized fragments are filtered by a mass analyzer (e.g., quadrupole, time-of-flight) that separates ions based on their mass-to-charge ratio (
m/z). - Detection and Data Acquisition: Detectors (electron multipliers or Faraday cups) count ions, generating a mass spectrum. Chromatograms plot ion abundance vs. retention time.
- Data Interpretation: Software compares spectral data to reference libraries (e.g., NIST) for compound identification and quantitation.
Technical Components and Schematic Diagram of GC-MS
A GC-MS system typically consists of the following critical components:- Injector Port: Allows precise sample introduction and vaporization.
- Gas Chromatograph Oven and Column: Maintains temperature-controlled environment for effective chromatographic separation.
- Carrier Gas Supply: High-purity helium or nitrogen transports analytes through the system.
- Transfer Line: Thermally insulated conduit connecting GC to MS, preventing analyte condensation.
- Ion Source: Electron Ionization (EI) chamber generating reproducible fragment ions.
- Mass Analyzer: Quadrupole or Time-of-Flight (TOF) filter separating ions by
m/z. - Detector: Records ion abundance, typically an electron multiplier.
- Data System: Software for data acquisition, spectrum processing, and library searching.
Sample Injector → GC Column (Oven) → Transfer Line → Ion Source (EI) → Mass Analyzer → Detector → Data SystemCritical Terms and Concepts Explained
- Retention Time
- The time elapsed from sample injection to the detector response for a given analyte; essential for chromatographic identification.
- Electron Ionization (EI)
- A hard ionization method that produces reproducible fragmentation patterns, critical for spectral library matching.
- Mass-to-Charge Ratio (
m/z) - The fundamental parameter in MS representing ion mass divided by its charge, enabling mass analysis.
- Eluent Fractions
- Segments of the chromatographic output collected over time, which can be directed for further analysis.
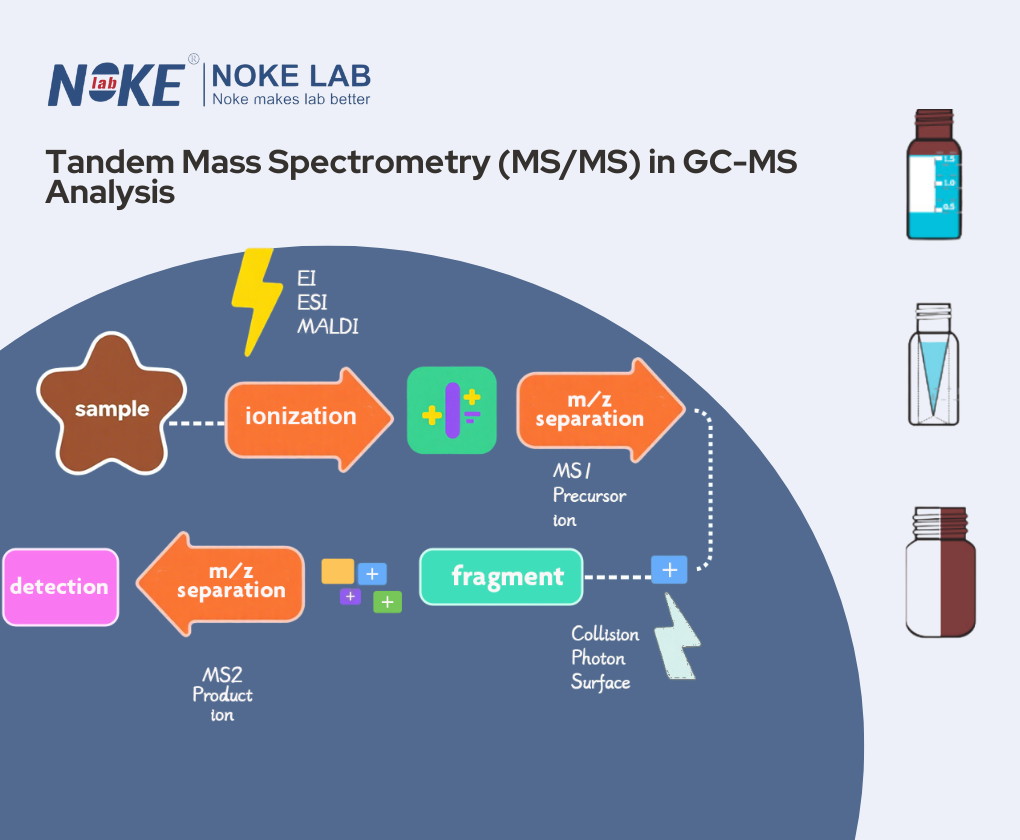
- Tandem Mass Spectrometry (MS/MS)
- A technique involving multiple stages of mass analysis for enhanced selectivity and sensitivity, often combined with GC-MS for complex matrices.
Applications Across Scientific Fields
GC-MS is extensively used in:- Environmental Monitoring: Detection of volatile organic compounds (VOCs), pesticides, and pollutants in air, water, and soil.
- Forensic Science: Identification of drugs, explosives, toxins, and arson accelerants.
- Pharmaceutical Industry: Drug development, pharmacokinetics, metabolite profiling, and quality control.
- Food and Flavor Industry: Analysis of flavor compounds, contaminants, and adulterants.
- Clinical and Biomedical Research: Biomarker discovery and metabolomics.
Advantages and Limitations
Advantages
- High sensitivity and selectivity for trace-level detection.
- Structural elucidation via reproducible fragmentation patterns.
- Wide range of analytes, especially volatile and semi-volatile compounds.
- Compatibility with tandem MS techniques (GC-MS/MS) for complex samples.
- Automated, high-throughput analysis with excellent reproducibility.
Limitations
- Limited to compounds that can be vaporized without decomposition.
- Requires sample preparation for non-volatile or thermally labile substances.
- Instrumentation and maintenance can be costly.
References and Further Reading
📚 Related Articles
Micro Size, Pure Keeps!
Tiny Parts. Mighty Manufacturing. PureChromatography Beyond.
Laboratório Noke